
Development Overview
The neural tube plays a key role in neural development during the early stages of embryogenesis. Cells located along Different parts of the vertebrate axis acquire different functions transiently as a result of the secretion of different chemical signals: morphogens.
Figure: showing the morphogens which are involved in patterning the anterior posterior axis of an embryo
During the fourth week of neurulation of an embryo, the rostral end of the spinal cord enlarges into three primary brain vesicles:
The prosencephalon, mesencephalon and the rhomencephalon.
It is the prosencephalon from which the primary vesicle then splits into the secondary vesicles: the telencephalon and the Diencephalon, these divisions occur during the fifth week .
Further subdivisions of the primary vesicles to their secondary vesicles can be seen below.
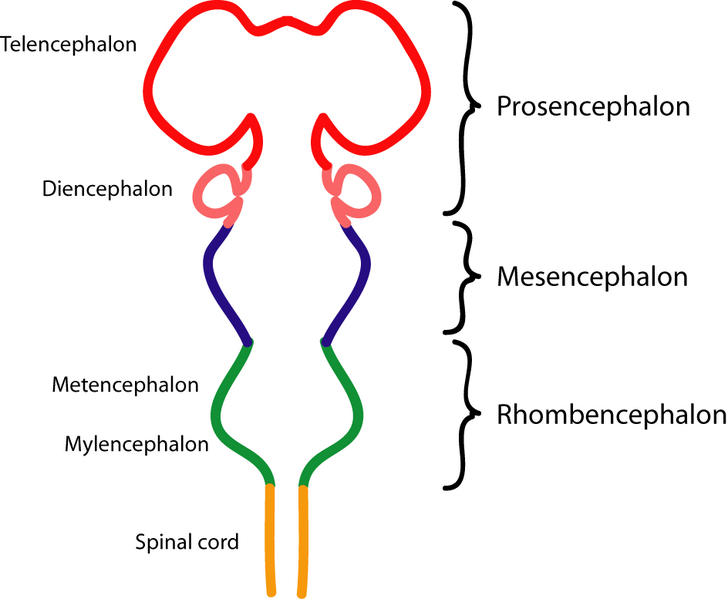
Image showing the development of the encephalon over time.Image adapted courtesy of commons.wikimedia.org/wiki/File:Encephalon.png
The diencephalon then further gives rise to the: thalamus and the hypothalamus
Morphogens involved in hypothalamic development
As previously described the brain and spinal cord transiently develops as a result of sequential activation of morphogens from cells in an autonomous and non-autonomous manner.
Much research has been done to establish how these events occur in the hypothalamus however further research is still required to grasp the morphogenesis of the hypothalamus in its entirety.
Studies carried out on chick embryos using methods such as fate mapping and overexpression by researchers have allowed us to understand the fundamentals of this process.
Because experiments cannot be carried out on human embryo, the time lag of all the events which occur below cannot be specified precisely however it does portray the order in which these events occur.
The time lags of these events however have been specified in chick embryos.
Illustration of all the different morphogens at different stages involved in patterning the hypothalamus and the initiation of the development of the infundibular stalk
Shh and nodal together are believed to initiate the differentiation of floor plate like cells at the ventral midline of the forebrain.
This source of Shh then also induces the secondary expression of Shh in basal plate cells.
An underlying tissue called the prechordal mesoderm which derives from the node during gastrulation turns on the morphogen BMP7.
BMP7 then upregulates the expression of a T-box transcription factor called Tbx2 in a concentration dependent manner from high concentration ventrally gradually decreasing dorsally along the hypothalamus.
Tbx2 is believed to completely downregulate the expression of shh over time in floor plate cells of the hypothalamus, however laterally shh remains actively secreted along with other morphogens eg.Pax6 and nkx2.2.
This compartmentalisation of the hypothalamus allows different parts of this structure to acquire different fates.
It is essential for shh to be downregulated in the floor plate of hypothalamic cells to allow the infundibular stalk and median eminence to then form from the ventral hypothalamus.
Sequentially the floor plate cells acquire new characteristics including the expression of FGF10 and BMP as the Tbx2 protein has now eliminated Shh signalling within this region of the hypothalamus, acting as a competence factor.
It is the inferior cells at the ventral midline of the hypothalamus which start expressing FGF10 and this protein is essential for the development of the infundibulum.
The FGF10 expressing cells remain at the ventral midline of the hypothalamus and induce circular rings of other gene expression in a concentric manner. These induce/maintain the expression of the genes; FGF3 and SOX3 which also derive from the tissue underlying the hypotholamus: the anterior floor plate.
The neuronal cells forming the walls of the infundibulum are then generated as a result of the expression of both FGF3 and SOX3 expressed ambiently around the ventral midline expression of FGF10. These proteins then induce the proliferation of new cells which then form the wall of the infundibulum thus the infundibulum is composed of two cell types: cells forming the floor and cells forming the wall.
Proteins expressed prior to these behave as signalling ligands in guiding the hypothalamo-pituitary axons:
FGF10 expressed at the ventral midline forming the prospective inferior cells of the pituitary gland, behaves as a chemoattractant.
Where as BMP, co-expressed from the floor plate surrounding the expression of FGF10 acts as a chemorepullsant. Taking both these factors into account, axons from the hypothalamus can then be guided via attractive/repulsive guidance cues to grow along the correct pathway down the infundibular stalk and reach their correct destination.